Research
Mechanical loading bioreactors for production of tissue engineered cartilage
| Principal investigator: | Martin KNIGHT |
| Funding source(s): | EPSRC |
| Start: 09-11-2000 / End: 10-11-2006 | |
| Directly incurred staff: | Martin Knight |
 Background and Context Articular cartilage provides a low friction, low wear bearing surface within synovial joints. The tissue has poor intrinsic repair properties due to its avascular nature and consequently cartilage damage progresses to debilitating arthritis with joint pain and stiffness. Existing repair techniques based on the Pridie method produce poor quality fibro-cartilage whilst total joint replacements have a limited lifespan particularly in younger patients. There is therefore a real clinical need for improved cartilage repair techniques. It is well established that physiological mechanical loading of articular cartilage causes the cells to modulate their synthesis and catabolism of the extracellular matrix. This ability of chondrocytes to sense and respond to mechanical loading is therefore critical to the health and function of the tissue. However, the underpinning mechanotransduction pathways are unclear. By determining these signalling pathways it may be possible to develop functional tissue engineered cartilage using specialised bioreactors to mechanically stimulate cells seeded within novel 3D scaffolds.
Background and Context Articular cartilage provides a low friction, low wear bearing surface within synovial joints. The tissue has poor intrinsic repair properties due to its avascular nature and consequently cartilage damage progresses to debilitating arthritis with joint pain and stiffness. Existing repair techniques based on the Pridie method produce poor quality fibro-cartilage whilst total joint replacements have a limited lifespan particularly in younger patients. There is therefore a real clinical need for improved cartilage repair techniques. It is well established that physiological mechanical loading of articular cartilage causes the cells to modulate their synthesis and catabolism of the extracellular matrix. This ability of chondrocytes to sense and respond to mechanical loading is therefore critical to the health and function of the tissue. However, the underpinning mechanotransduction pathways are unclear. By determining these signalling pathways it may be possible to develop functional tissue engineered cartilage using specialised bioreactors to mechanically stimulate cells seeded within novel 3D scaffolds.
 Executive Summary
Executive Summary
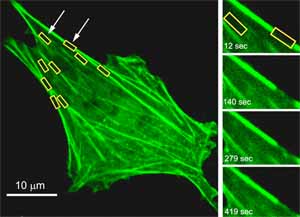
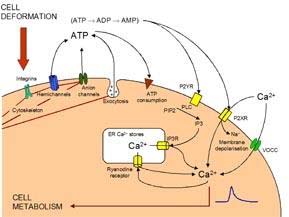
The Fellowship successfully met, to a greater or lesser extent, all 4 of its main objectives as follows: 1. Develop a new scaffold biomaterial for cartilage repair. 2. Develop a bioreactor for mechanical stimulation. 3. Formulate a hypothesis for chondrocyte mechanotransduction in cell seeded scaffolds. 4. Optimise in vitro mechanical conditioning parameters for cell seeded cartilage repairs. The work associated with these objectives is discussed in the following sections. However, in the case of objective 3, a strategic decision was made to go into greater depth in this fundamental area of mechanotransduction and mechanobiology. In deed, during the course of the Fellowship, Dr Knight has become an internationally recognised researcher in this field, publishing 3 book chapters and over 20 journal papers [1-22]. Exciting research has elucidated a mechanotransduction intracellular calcium signalling pathway triggered by the release of ATP which binds to purine receptors on the chondrocyte cell membrane. This pathway is activated by cyclic compression and modulated by strain rate but not frequency or number of cycles. The pathway stimulates downstream up-regulation of proteoglycan synthesis and cell proliferation providing potential therapeutic targets for pharmaceutical manipulation. Mechanical loading also triggered remodelling of the cortical actin cytoskeleton, possibly via a calcium dependent pathway, which reduces cell modulus. This mechanism may allow the cell to distort without damage, since inhibition of calcium-induced actin remodelling causes a load-induced inflammatory response with increased nitric oxide synthesis. However, loading was also associated with alterations in gene expression for actin-associated proteins, thereby providing a further mechanism for cytoskeletal remodelling and alterations in mechanosensitivity. Overall, this increased understanding of chondrocyte mechanobiology together with expertise in bioreactor design and the use of novel silk-based scaffolds, provides exciting opportunity for new cartilage tissue engineered repair strategies.

