Research
The Synergistic Effect of Bone Graft Scaffold Architecture and Mechanical Environment on hMSCs Responses in vitro
| Principal investigator: | Karin HING |
| Co-investigator(s): | S Rawlinson (SMD) |
| Funding source(s): | CSC |
| Start: 15-09-2014 / End: 16-09-2018 | |
| Directly incurred staff: | Feng Yang |
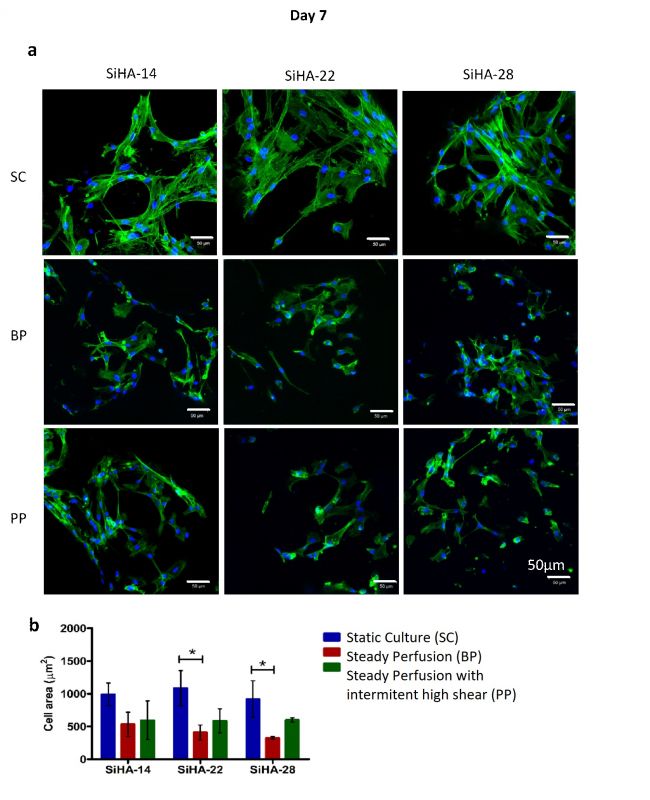
Porous silicon substituted hydroxyapatite (SiHA) as bone graft scaffold, especially those having high micro-porosity, show excellent bone ingrowth and repair in vivo. It is accepted that the mechanical environment to which cells are exposed regulates cellular differentiation and bone tissue regeneration. One mechanism by which scaffold architecture may regulate bone formation in situ could be through influencing the fluid shear stress distribution of interstitial fluid. Therefore, the aim of this study was to investigate the combined influence of scaffold architecture and fluid shear environment on human mesenchymal stem cells (hMSCs) responses.
In order to achieve this, SiHA scaffolds with systematically varied levels of macro and micro (or strut) porosity were synthesised, characterised, and used in in vitro experiments. A 3D in-house perfusion bioreactor system was established, and hMSC seeded scaffolds were exposed to static conditions (SC), steady perfusion conditions (BP) at a flow rate of 0.07 ml.min-1 or steady perfusion conditions with one hour daily at an elevated flow rate of 2.5ml.min-1. The cytoskeleton of hMSCs was reorganized under both types of perfusion conditions compared with static conditions, where perfuison induced both ERK1/2 and pEKR1/2 translocation from the cytoplasm to nucleus. However, hMSCs cultured under steady perfusion conditions were observed to differentiate towards an osteogenic lineage, while daily exposure to one hour at an elevated flow rate appeared to induce hMSCs to differentiate towards a chondrogenic lineage. Gene expression of osteogenic or chondrogenic markers were not sensitive to scaffold micro-porosity under static conditions. However, the expression of osteogenic transcription factor OSX increased significantly with increasing scaffold micro-porosity under steady perfusion conditions. These results demonstrated that selection of appropriate culture conditions is key to the evaulation of graft materials in vitro.

