Research
Mechanical loading modulates cytoskeletal organisation in living chondrocytes
| Principal investigator: | Martin KNIGHT |
| Co-investigator(s): | R.H. de Silva (UCL) |
| Funding source(s): | BBSRC |
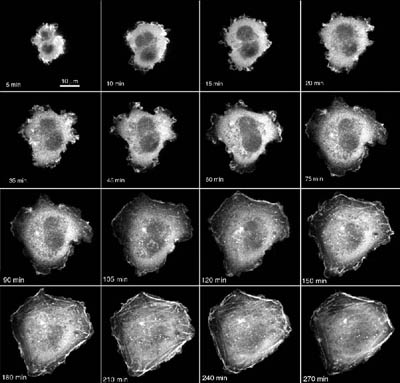 Background and Context
Background and Context
Articular cartilage provides a low friction, low wear bearing surface within synovial joints. Existing approaches for treatment of cartilage injury or disease have poor long term functionality, particularly in younger patients. There is therefore a real clinical need for improved cartilage repair techniques. It is well established that physiological mechanical loading of articular cartilage causes the cells to modulate their synthesis and catabolism of the extracellular matrix. This ability of chondrocytes to sense and respond to mechanical loading is critical to the health and function of the tissue. There is now increasing interest in the application of mechanical conditioning to improve the quality of tissue engineered cartilage repair. However in order to optimise this approach it will be necessary to elucidate the underlying mechanotransduction pathways.
The cytoskeleton, consisting of actin microfilaments, microtubules and vimentin intermediate filaments, is of fundamental importance in the control of many aspects of cell biology. Much of the functionality of the cytoskeletal networks lies in their ability to constantly remodel in response to changing stimuli. The cytoskeleton has been implicated in mechanotransduction within a variety of cell types. Nevertheless, little is known of the effect of compressive loading directly on the cytoskeleton within living chondrocytes. This proposal examines the influences of compressive strain on displacement, deformation and subsequent remodelling of the cytoskeleton and the signalling pathways involved.
Although this proposal is focused on the cytoskeleton within articular chondrocytes, many of the findings are fundamental to a range of cell types and tissues.
Executive Summary
The Research Grant successfully met, to a greater or lesser extent, all of its main objectives in examining the behaviour of the cytoskeleton in cartilage cells subjected to compression. The majority of studies focussed on the effect of mechanical forces on actin microfilaments as these have been especially implicated in mechanotransduction and cell mechanics and are known to remodel in response to mechanical stimuli in a variety of other cell types. A specially-designed loading rig mounted on the stage of an inverted microscope was used to compress chondrocytes cultured within 3D agarose gels. In addition, micropipette aspiration techniques were used to apply localised cell distortion and to examine the relationship between cytoskeletal remodelling and cell mechanics. Due to the lack of appropriate fluorescent stains to visualise the cytoskeleton in living cells, chondrocytes were transfected with enhanced green fluorescent protein (eGFP) attached to one of the cytoskeletal proteins. Successful eGFP plasmids were created for actin, tubulin and vimentin. A computational digital image correlation technique was developed and used to quantify the displacement and deformation on the cytoskeletal features with sub-pixel resolution based on confocal images. Results have been combined with computational modelling to elucidate aspects of intracellular and cellular mechanics. In addition fluorescent recovery after photobleaching (FRAP) techniques were optimised for quantifying cytoskeletal dynamics in specific regions of the cell. Results revealed how mechanical loading triggers distortion and rapid remodelling of the cortical actin cytoskeleton, via a calcium dependent pathway, which reduces cell modulus. This mechanism may allow the cell to distort without damage, since inhibition of the calcium-mediated actin remodelling causes a load-induced inflammatory response with increased nitric oxide synthesis. However, more prolonged loading was associated with alterations in gene expression for actin-associated proteins, thereby providing a further pathway for cytoskeletal remodelling.
Overall the study revealed that the chondrocyte cytoskeleton is sensitive to its mechanical environment and will remodel in response to changing mechanical loading thereby producing alterations in cell mechanics, mechanotransduction and mechanosensitivity.

