Research
The role of membrane-actin adhesion in regulating stem cell viscoelastic properties and blebability during differentiation
| Principal investigator: | Martin KNIGHT |
| Co-investigator(s): | David LEE |
| Funding source(s): | EPSRC |
| Start: 09-01-2012 / End: 10-01-2015 | |
| Directly incurred staff: | Kristina Sliogeryte |
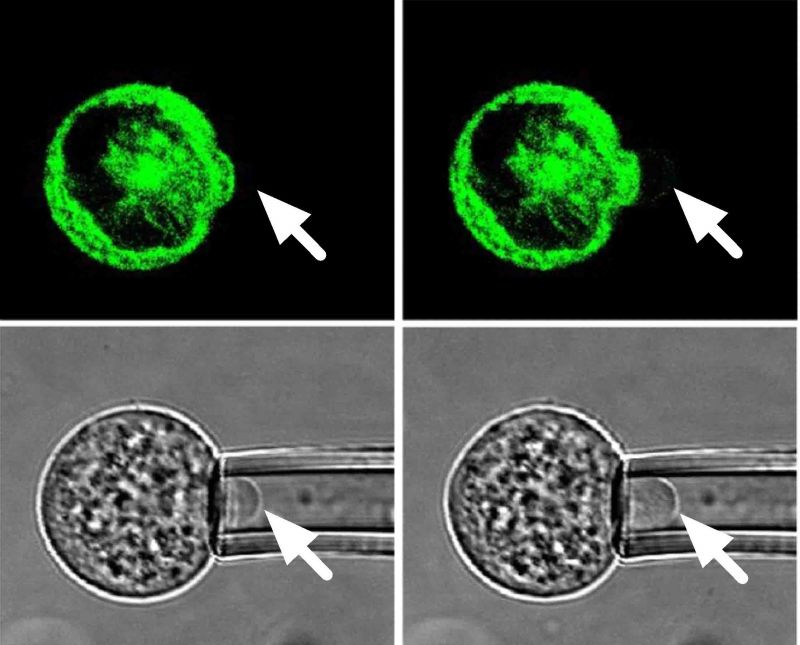 This PhD examines how chondrogenic differentiation of human mesenchymal stem cells (hMSCs) regulates the interaction between the cell membrane and the actin cortex, thereby controlling cell biomechanics. The thesis also investigates the viscoelastic properties of primary articular chondrocytes and the effect of de-differentiation. Micropipette aspiration was used to measure the pressure required for membrane-cortex detachment as well as the apparent equilibrium and instantaneous moduli based on fitting the standard linear solid model to the temporal changes in aspiration length induced by a step negative pressure. Simultaneous live cell confocal imaging of actin dynamics was achieved by transfecting cells with LifeAct-GFP.
This PhD examines how chondrogenic differentiation of human mesenchymal stem cells (hMSCs) regulates the interaction between the cell membrane and the actin cortex, thereby controlling cell biomechanics. The thesis also investigates the viscoelastic properties of primary articular chondrocytes and the effect of de-differentiation. Micropipette aspiration was used to measure the pressure required for membrane-cortex detachment as well as the apparent equilibrium and instantaneous moduli based on fitting the standard linear solid model to the temporal changes in aspiration length induced by a step negative pressure. Simultaneous live cell confocal imaging of actin dynamics was achieved by transfecting cells with LifeAct-GFP.
The studies herein demonstrate that the strength of the membrane-cortex adhesion increased from 0.15kPa in stem cells to 0.71kPa following chondrogenic differentiation of hMSCs. This effect was associated with a reduced susceptibility to mechanical and osmotic bleb formation and an increase in the apparent modulus of the differentiated stem cells as well as reduced cell migration. Differentiated stem cells expressed greater levels of the membrane-cortex ERM (ezrin, radixin and moesin) linker proteins at both gene and protein level. Transfection of undifferentiated stem cells with dominant active ezrin-T567D-GFP increased the strength of the membrane-cortex bond. This suggests that increased expression of ERM in differentiated cells is responsible for the reduced blebability and increased modulus observed. Differentiated cells also exhibited greater F-actin density and slower actin remodelling, based on FRAP analysis of cells transfected with LifeAct-GFP, which may also influence cellular viscoelastic properties. Finally the thesis demonstrates that dedifferentiation of primary chondrocytes also increased F-actin density and expression of ERM linker proteins with associated alterations in membrane-actin adhesion and cellular viscoelastic properties. In summary this study provides new insights into the role of membrane-actin cortex adhesion and the expression of ERM linker proteins in regulating the mechanical properties of chondrocytes and mesenchymal stems cells.

