Research
The role of mechanical loading in modulating chondrocyte hedgehog signalling via primary cilia.
| Principal investigator: | Martin KNIGHT |
| Co-investigator(s): | P. Chapple |
| Funding source(s): | BBSRC |
| Start: 01-10-2009 / End: 30-09-2013 | |
| Directly incurred staff: | Clare Thompson |
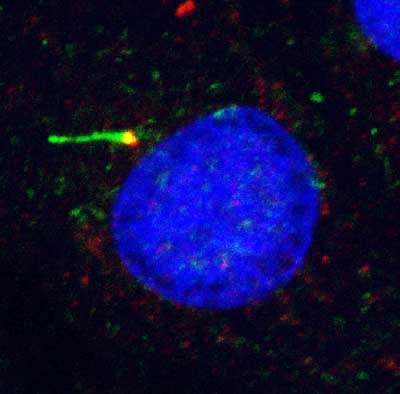 Background
Background
Chondrocytes express primary cilia consisting of a membrane coated axoneme which projects into the pericellular matrix and an intracellular basal body that comprises the most mature of the two centrioles. The function of this organelle in cartilage is unknown, however recent studies indicate that the primary cilium is involved in hedgehog (Hh) signalling which regulates chondrocyte proliferation and differentiation in the growth plate. Consequently lack of cilia and associated absence of smoothened-patched (Smo-Ptc) Hh signalling complexes on the cilium, causes dwarfism by accelerating the onset of terminal hypertrophy. In addition, the chondrocyte primary cilium may also function as a mechanoreceptor, as in other cell types where deflection of the cilium initiates intracellular Ca2+ signalling as part of a mechanotransduction signalling cascade. Studies from Knight’s group have demonstrated that mechanical loading also activates Ca2+ signalling in chondrocytes and that key components of this signalling pathway are present on the primary cilium. This collaborative interdisciplinary proposal brings together an expert in cilia-Hh signalling (Chapple) and an expert in chondrocyte mechanobiology and primary cilia (Knight). The PhD student will examine the interrelationship between the two putative functions of the primary cilium by testing the following hypothesis.
Hypothesis: Significant interplay exists between chondrocyte Hh signalling and mechanotransduction both of which are mediated by the primary cilium.
Objectives and Programme of Work: The research will focus on the following objectives:
1) Characterise chondrocyte Hh signalling and how the associated proteins are localised on the cilium,
2) Determine the influence of mechanical loading on Hh signalling, and
3) Determine the influence of Hh signalling on mechanotransduction pathways involving the primary cilium.
An established murine articular chondrocyte cell line (H5) will be used with cells embedded within agarose gel in order to facilitate physiological mechanical loading based upon protocols and test rigs extensively used in the Knight lab. Preliminary studies have shown that these cells are ciliated in a similar manner to primary cells in situ. Activation of Hh signalling in articular chondrocytes will be assessed using a luciferase based reporter for Gli1 transcription factor activity. Cells will be stimulated with Hh agonists, or conditioned media from an existing stable cell line expressing sonic Hh. Cellular levels of the Ptc Hh receptor, the expression of which is rapidly unregulated upon activation of Hh signalling, will be monitored by real time PCR and western analysis. Ciliary axoneme localisation of the downstream Hh protein smo, increases upon signal transduction. We will therefore use immunofluorescent staining and subsequent confocal microscopy to monitor smoothened localisation after stimulation with ligand. Most of these assays are already established for other ciliated cell lines in the Chapple laboratory. To investigate the interplay between mechanotransduction and Hh signalling, cells will be subjected to cyclic compression with and without ligand stimulation of Hh signalling. Studies will therefore examine whether the mechanotransduction pathway, is modulated by activation of chondrocyte Hh signalling. This will involve quantification of ATP release, Ca2+ signalling, nitric oxide synthesis and downstream metabolic events such as proteoglycan synthesis following established protocols. In addition the influence of loading on the Hh signalling pathway will be determined as above. Results will be compared with unloaded controls. Hh signalling will also be examined following inhibition of cilia-mediated mechanotransduction using a range of antagonists, previously characterised in the Knight lab (e.g. gadolinium, flufenamic acid, suramin, thapsigargin).

