Research
Viscoelastic biomechanics behaviour of living cells at a cellular and subcellular level
| Principal investigator: | Martin KNIGHT |
| Co-investigator(s): |
| Funding source(s): | EPSRC |
| Start: 01-10-2009 / End: 02-10-2012 | |
| Directly incurred staff: | Priyanka Pravincumar |
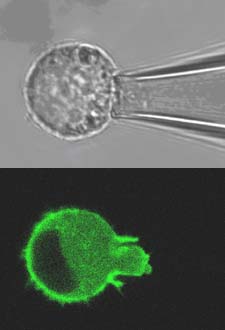 The biomechanics of living cells and their response to mechanical forces is critical to the function and health of a variety of tissues including articular cartilage. This field of mechanobiology therefore has enormous potential to be exploited in the evaluation of pharmacological agents and the development of tissue engineering strategies for the regeneration of diseased or damaged tissue. Consequently, the key strategic aim of this proposal is to develop methods to quantify the biomechanical properties of cartilage cells, termed chondrocytes, and to use these methods to examine a series of fundamental test hypotheses.
The biomechanics of living cells and their response to mechanical forces is critical to the function and health of a variety of tissues including articular cartilage. This field of mechanobiology therefore has enormous potential to be exploited in the evaluation of pharmacological agents and the development of tissue engineering strategies for the regeneration of diseased or damaged tissue. Consequently, the key strategic aim of this proposal is to develop methods to quantify the biomechanical properties of cartilage cells, termed chondrocytes, and to use these methods to examine a series of fundamental test hypotheses.
For these studies, the viscoelastic time-dependent behaviour of isolated viable chondrocytes will be determined using micropipette aspiration. This technique involves partially aspirating a single cell into a glass micropipette at known suction pressures (typically 1-5cm H2O) and simultaneously visualising the cell using confocal or multiphoton microscopy (Fig 1). Measurements of aspiration length into the pipette can then be used to derive cellular biomechanical viscoelastic properties based on theoretical models. By combining this approach with fluorescent labelling of intracellular structures such as the nucleus and the cytoskeletal networks, it is possible to build up an understanding of sub-cellular strain transfer functions and intracellular biomechanics as well as potential mechanotransduction pathways such as alterations in gene transcription associated with nucleus deformation. In particular, the study will examine the deformation and remodelling of the actin cytoskeleton which forms a dynamic cortical network just beneath the cell membrane and provides structural integrity to the cell.
Visualisation of actin will be achieved using transfection with a GFP-actin DNA plasmid and confocal microscopy with associated fluorescence recovery after photobleaching (FRAP) techniques to quantify actin dynamics and remodelling.

