Centre for Bioengineering
Investigating the cardiomyocyte rigidity sensing mechanism with micro patterned surfaces and nanopil
| Funding source(s): | BBSRC Biotechnology and Biological Sciences Research Council |
| | Start: 01-02-2019 / End: 31-03-2023 |
| | Amount: £490545 |
| Research Centre: | |
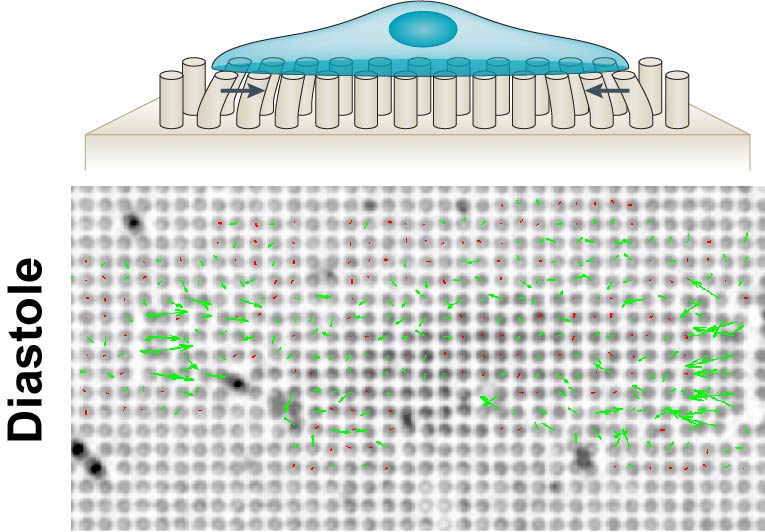
While chemical cues have well-established roles in guiding cellular processes from migration to differentiation or cell death, there is growing evidence of a role for mechanical stimuli. This has also been proposed for the cells in the heart, where changes to the extracellular matrix result in stiffening of the cellular microenvironment during development - and even further in heart disease.
Our previous results indicated cardiomyocytes simultaneously sense the contractions of muscle and non-muscle myosin. Depending on the activity of each class of myosin motors, this will lead to different dynamics of stretching of the mechanosensitive protein talin. Moreover, our results suggest that talin stretching feeds back into integrin activation and downstream actin assembly through the formin FHOD1.
Because there is competition between different actin assembly proteins this will impact the actin nucleation through other proteins and as a result the maturity of the contractile structures. However, the (inter-) regulation of the cardiomyocyte actin assembly in response to stiffness is still unclear. Moreover, based on the literature and our own results we hypothesise that not only the stiffness of the heart, but also the composition of the adhesions will change during development and disease, with important effects on how the mechanical sensing is regulated.
To answer these critical questions, we will use here a bottom up approach based on micropatterning to analyse adhesion composition and the cooperation - or competition between different actin nucleators in stem cell derived cardiomyocytes of different maturity, or genetic background. Moreover, we will use nanopillars and molecular tension sensors to analyse cardiomyocyte forces on different stiffness, extracellular matrix composition or after manipulation of key mechanosensor proteins. We expect this approach to result in a comprehensive understanding how cardiomyocytes sense the rigidity of their environment.


