Research
Peptide Self-assembly
| Principal investigator: |
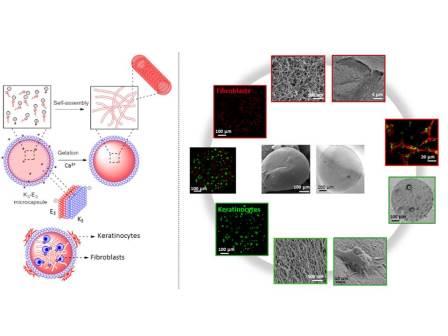 Having the flexibility of using 20 chemically different amino acids, peptides are versatile assembly components due to the intrinsic functional diversity of amino acids. In addition, a number of very important physiological and biochemical functions of life are influenced by peptides. For example, peptides are involved in receptor-mediated signal transduction, influencing cell-cell communication upon interaction with receptors. As self-assembling building blocks, peptides are readily accessible through chemical synthesis; the information required for their self-assembly is encoded within their sequence; their self-assembly is usually spontaneous (simple method to develop nanostructured materials), instantaneous (milliseconds) and reproducible (defined stable structures). Furthermore, by varying systematically the chemical structure (e.g. sequence size and nature of the amino acid side groups) during synthesis it is possible to adjust the self-assembling properties of the building blocks as well as to produce a variety of diverse nanostructures (e.g. micelles, fibers, vesicles). The function of self-assembling peptides clearly depends on their structural properties and this structural information is important to understand the mechanism of peptide self-assembly and determine potential applications.
Having the flexibility of using 20 chemically different amino acids, peptides are versatile assembly components due to the intrinsic functional diversity of amino acids. In addition, a number of very important physiological and biochemical functions of life are influenced by peptides. For example, peptides are involved in receptor-mediated signal transduction, influencing cell-cell communication upon interaction with receptors. As self-assembling building blocks, peptides are readily accessible through chemical synthesis; the information required for their self-assembly is encoded within their sequence; their self-assembly is usually spontaneous (simple method to develop nanostructured materials), instantaneous (milliseconds) and reproducible (defined stable structures). Furthermore, by varying systematically the chemical structure (e.g. sequence size and nature of the amino acid side groups) during synthesis it is possible to adjust the self-assembling properties of the building blocks as well as to produce a variety of diverse nanostructures (e.g. micelles, fibers, vesicles). The function of self-assembling peptides clearly depends on their structural properties and this structural information is important to understand the mechanism of peptide self-assembly and determine potential applications.

