Dr Caroline Roney
MMath, MRes, PhD
Research Overview
Computational Medicine, Cardiac Arrhythmia, Biophysical Modelling, Machine learning, Signal Processing, Image Processing
Interests
Our research uses personalised atrial models and virtual cohorts to design patient-specific treatment approaches for atrial fibrillation. We work with signal processing, mechanistic simulations and machine learning techniques for both basic science and clinical data. Some of our research applications are as follows.
Signal Processing of Cardiac Arrhythmia Data:
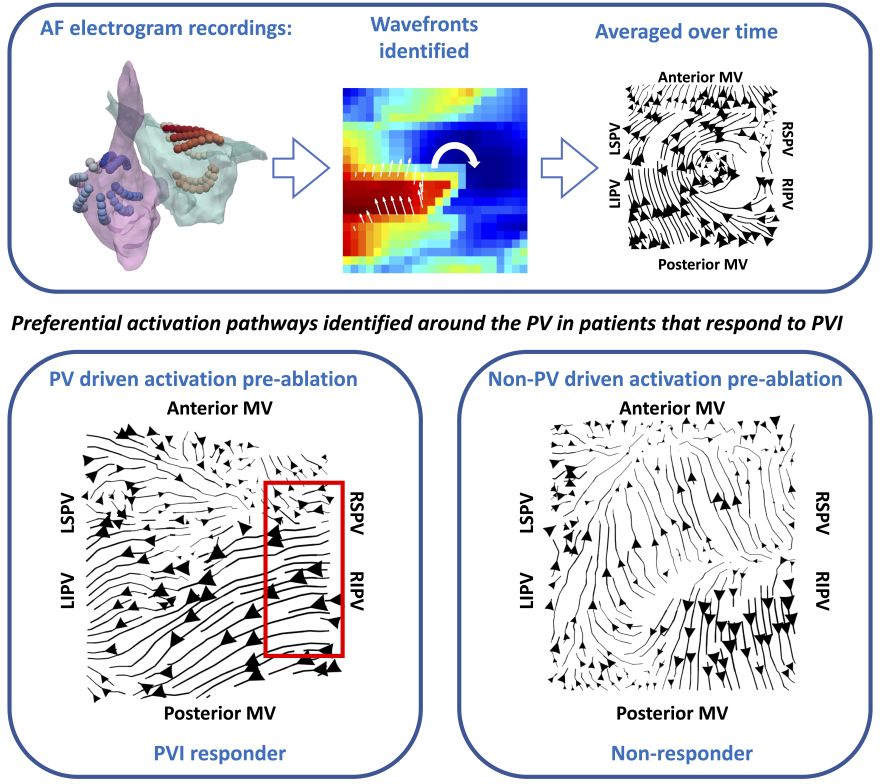
Cardiac conduction properties exhibit large variability between patients and affect patient-specific arrhythmia mechanisms. We developed and compared multiple methodologies for calculating the conduction velocity of the propagation wavefront, for phase mapping of atrial fibrillation data and for identifying preferential pathways of activation. We recently used these techniques to predict acute response to catheter ablation therapy.
Constructing patient-specific atrial models:
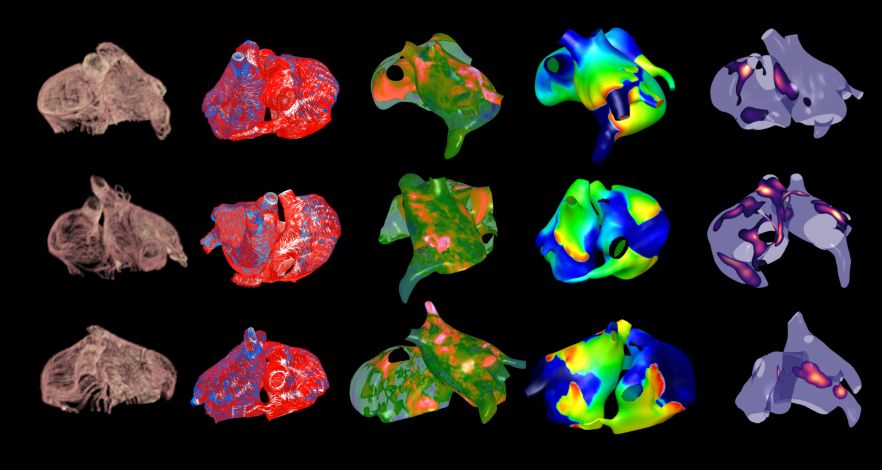
We have developed a pipeline for constructing patient-specific biophysical models from imaging data. These models include atrial fibres, fibrotic remodelling, and personalised electrophysiology. We simulate atrial fibrillation and identify critical regions that could be targeted during catheter ablation therapy.
Atrial fibre modelling:
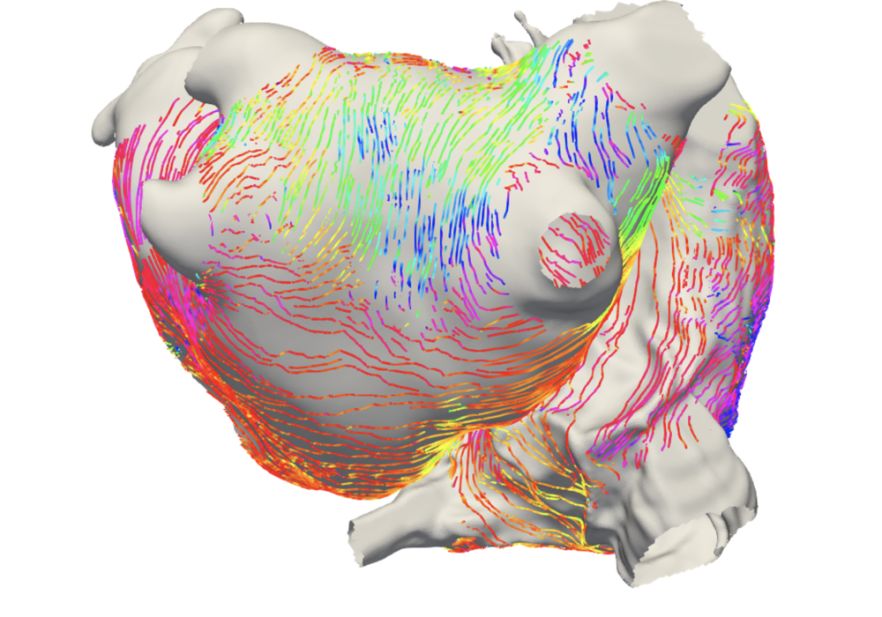
Atrial anisotropy and fibre direction affects electrical propagation patterns, anchor locations of atrial reentrant drivers, and atrial mechanics. However, it is challenging to clinically measure electrical anisotropy and patient-specific atrial fibre fields are not available. To investigate electrical anisotropy, we developed a technique to estimate conduction anisotropy and fibre direction from clinically available electrical recordings. To investigate structural anisotropy, we constructed an atrial fibre atlas from a high-resolution DTMRI dataset and developed a methodology for automatically assigning fibres to patient-specific anatomies.
Modelling Atrial Fibrosis:
Atrial fibrosis plays an important role in both the initiation and maintenance of atrial fibrillation. Fibrotic remodelling is multifactorial and there are multiple methodologies for incorporating the effects of fibrosis in computational models. We demonstrated that the specific representation of fibrosis in biophysical atrial models has a large effect on rotor dynamics and needs to be carefully considered for patient specific modelling.
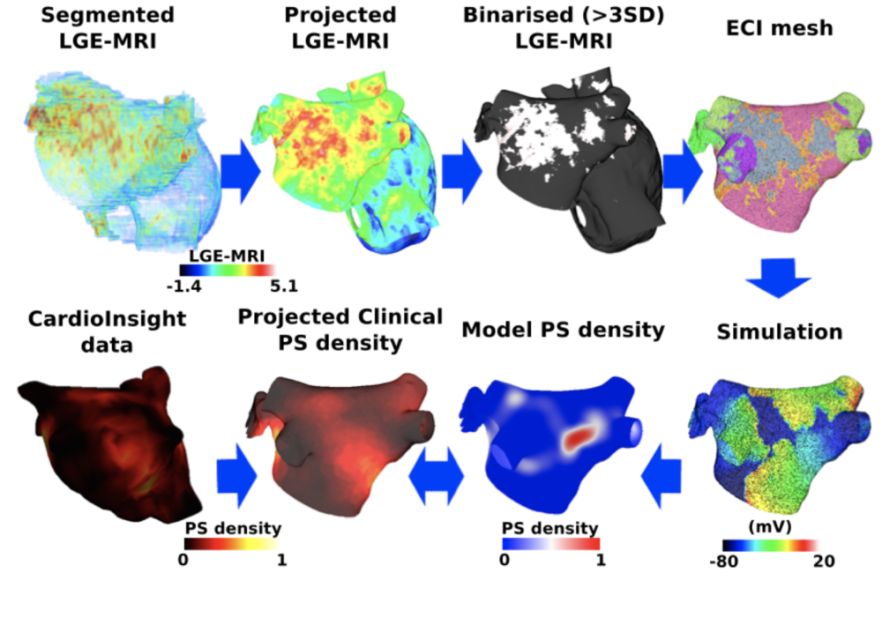
Schematic showing pipeline used for investigating the effects of modelling methodology of atrial fibrosis on driver dynamics.
Biophysical simulation and machine learning to predict response to ablation therapy using virtual patient cohorts
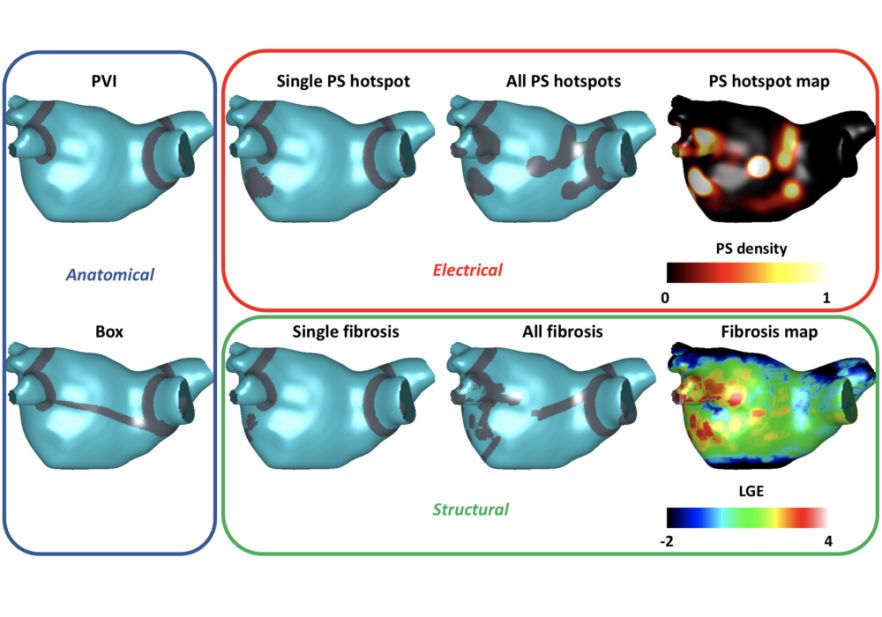
Catheter ablation therapy for persistent atrial fibrillation typically includes pulmonary vein isolation and may include additional lesions that target patient-specific anatomical, electrical or structural features. We aim to compare ablation techniques across virtual cohorts of atrial fibrillation patients to predict patient-specific outcome and design optimal ablation approaches.
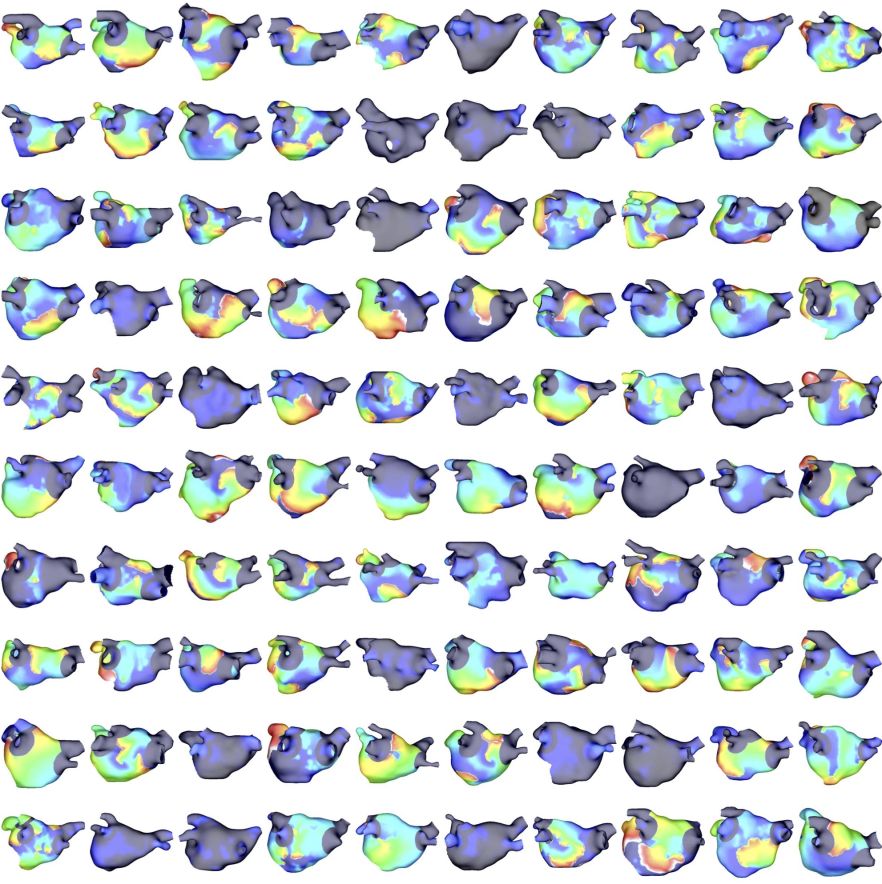
Applying pulmonary vein isolation results in different response across a cohort of patient-specific models. We compare simulated response to clinical patient outcome.
We combine biophysical simulations with machine learning techniques to predict either the acute response to therapy, or the longer-term response including whether atrial fibrillation recurs.