News
Queen Mary collaboration proposes a new adipo-mimetic model to study cancer cell migration in adipose tissues
22 July 2025

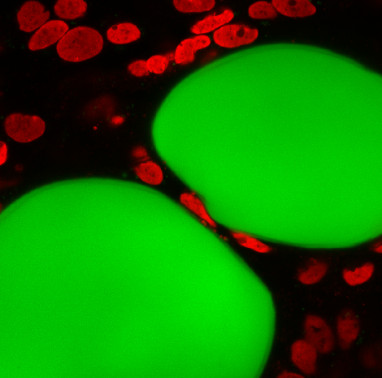
As there is increasing evidence correlating the progression of cancers such as ovarian and breast cancers or leukaemia with the microstructure and composition of their surrounding adipose (fat) tissues, this report, published in Nature Communications, shows that cancer cells migrate particularly in fat tissues and that this process is facilitated by the unique mechanical properties of these tissues.
The research, funded by the European Research Council, was conducted by a team from Prof Julien Gautrot’s Biointerface Laboratory and Centre for Predictive in-vitro Models at Queen Mary University of London, in collaboration with Professor Fran Balkwill of Barts Cancer Institute and Prof Kaisa Lehti and Dr Sahar Salehi at Karolinska Institutet in Sweden.
To study cancer cell migration in adipose tissues relevant to patients, the researchers characterised the speed at which ovarian cancer cells migrate through ovarian cancer patient adipose (fat) tissue samples and in a new type of biomaterials mimicking adipose tissues. In the latter adipo-mimetic biomaterials, they were able to control a range of different physio-chemical and biochemical parameters that together regulate cancer cell migration. In these adipo-mimetic biomaterials, they mimicked the microstructure and mechanics of fat cells (adipocytes) using microdroplets. This allowed them to vary the size and numbers of adipocyte-mimicking microdroplets in our biomaterials, parameters that correlate with the progression of cancers such as ovarian cancer and leukaemia. It also allowed them to control the local mechanics of the adipo-mimetic materials, to mimic that of fat tissues.
They found that the unique local mechanics of fat tissues and adipo-mimetic biomaterials, which are tightly packed cells, enable the rapid migration of cancer cells, without inducing significant nuclear damage and cell death.
This is the first time that a bioengineering approach has allowed scientists to recreate adipose tissues with such level of control over microstructural, mechanical and biochemical properties. How cancer cells would respond to these factors was not previously known.
Prof Julien Gautrot said "In many instances, cancer cells migrate and home in to adipose tissues, where they can form tumours. Surprisingly, very little is known of cell or cancer cell migration in adipose tissues and our work shows that this is regulated very differently to cell migration in other fibrous tissues. Understanding this phenomenon will be important, not only for the modelling of these processes in vitro, but also to identify novel strategies that may help us to fight the progression of cancers."
The correlations observed in the models developed, tissues obtained from patients and the established changes in tissue properties recovered from patients with different grades of ovarian cancers are particularly striking and suggest that adipose tissue may constitute a unique environment in which several types of tumours can develop.
| Contact: | Julien Gautrot |
| Email: | j.gautrot@qmul.ac.uk |
| Website: | |
| People: | Julien GAUTROT |
| Research Centre: | Bioengineering |




