News
New state-of-the-art microscope to support research in the School of Engineering and Materials Science
20 May 2020
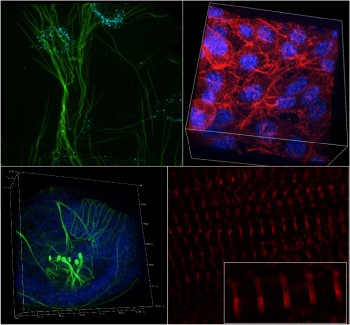
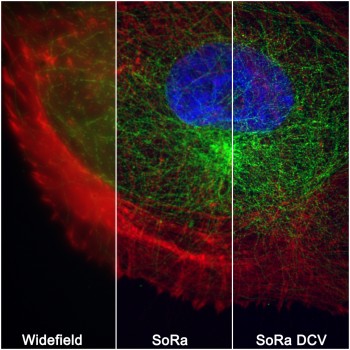
Queen Mary underlined its strong commitment to the ongoing research in SEMS by investing into a £375K state-of-the-art super-resolution spinning disc microscope.
Microscopy imaging approaches have long been a major research strength within the school, facilitating new collaborative links and supporting significant research funding and outputs in leading journals. The ability to conduct state-of-the-art imaging alongside our cell culture, mechanobiology and biomaterials labs in SEMS has been key to this success. The new microscope will further strengthen our position by offering fast, live, 3D super-resolution imaging with photomanipulation (FRAP, photoactivation) as well as fast, ratiometric FRET imaging with its two-camera setup.
This will enhance ongoing and enable future ground breaking research in areas including the examination of the effects of shear flow to understand the process of mechanoregulation of atherosclerosis; imaging of protein dynamics on primary cilia to identify new treatments for genetic ciliopathies, cancer and osteoarthritis; visualisation of particle movement and deformation within microfluidic systems; imaging of cell migration and movement within organ-on-a-chip models of bone metastasis; or to investigate the mechanobiology of contractile cardiomyocytes or engineered heart tissues to better understand and treat heart disease.
The microscope will be installed shortly and available for researchers from across the college.
| Contact: | Thomas Iskratsch |
| People: | Thomas ISKRATSCH Martin KNIGHT |
| Research Centre: | Bioengineering |
Updated by: Thomas Iskratsch




