News
Featured in Soft Matter: measuring the barrier adsorption/desorption rate of alohols on water
18 February 2019
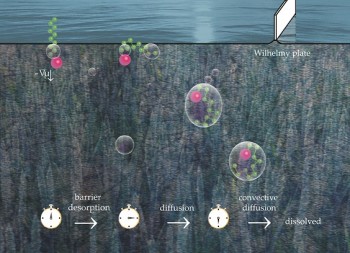
Featured in Soft Matter: decanol and dodecanol desorb from the surface of water following mixed barrier–diffusion kinetics.
The rate of adsorption/desorption of small molecules is generally assumed to proceed under diffusion control; the rate of the flip-flop like attachment/detachment is considered extremely fast. Well guess what? Even molecules as small as decanol have to overcome a significant barrier, tens of kJ/mol, to desorb. Morover, intensive convection can accelerate the diffusion transport of these surfactant to make the adsorption/desorption under complete barrier control!
This might sound surprising, but has actually been discovered already in 1975 by Baret et al., JCIS 53:50. The contribution of our work is in the greatly improved precision and detail: we were able to measure accurately the desorption rate as function of temperature and the compression of the surfactant monolayer. We demonstrate its phase-specificity. The data demonstrates that the desorption rate follows a simple dependance on the area per molecule: 1/(area x rate) ~ 1/area.
This might sound surprising, but has actually been discovered already in 1975 by Baret et al., JCIS 53:50. The contribution of our work is in the greatly improved precision and detail: we were able to measure accurately the desorption rate as function of temperature and the compression of the surfactant monolayer. We demonstrate its phase-specificity. The data demonstrates that the desorption rate follows a simple dependance on the area per molecule: 1/(area x rate) ~ 1/area.
| Contact: | Radomir I. Slavchov |
| Email: | r.slavchov@qmul.ac.uk |
| Website: | |
| People: | Radomir SLAVCHOV |
Updated by: Radomir Slavchov

