News
New research into the function of cardiac mechanosensing published in Science Advances
8 September 2025
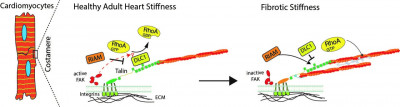
Recent discoveries have shown that cells in the heart are guided by the stiffness of their environment in a process, called mechanosensing. This influences the way they behave and function. Importantly, the stiffness of the heart changes in disease, however, it is still unclear how this contributes to the development of heart failure.
The group of Prof Thomas Iskratsch previously found that talin, a protein at interaction sites of the cells with the extracellular environment, is differentially stretched depending on the stiffness of their surrounding.
This new research, with Dr Emilie Marhuenda and Dr Ioannis Xanthis as first authors, and funded by the British Heart Foundation, shows this is leading to changes in the interaction partners of talin.
Importantly, the group found that this mechanism can be used by cells as a tool to remember the mechanical properties of the environment they have been sitting in. Such mechanical memory can fix cells in a state, which is useful for maintaining a healthy heart but if imprinted incorrectly can over time lead to heart cells being poorly adapted to their environment. This can lead to an aggravation of the disease.
Using different state-of-the art techniques, they characterised the interactions in vitro and in vivo (e.g. co-immunoprecipitation experiments, fluorescence recovery after photobleaching (FRAP), or in situ proximity ligation assays), and manipulated the interactions usingoptogenetic LOVTRAP assays.
The team found that the molecules DLC1, RIAM and Paxillin all bind to talin at different stiffness and that this interaction is preserved even in absence of tension. This shows that there is mechanical memory which is regulated through phosphorylation pathways. The research highlights DCL1 as a critical molecule in the heart cells which regulates RhoA activity at the cardiomyocyte adhesions. It’s loss of function leads to functional defects in the cardiomyocytes.
"We didn’t expect that the interactions would be persevered in absence of tension, hence we followed up on the finding to characterise the mechanical memory.” said Prof Iskratsch.
"Treatment of extracellular matrix stiffness has shown partial improvements of heart function but could not reverse the effects on the cardiomyocyte phenotype. Our results show that extracellular matrix stiffness and mechanical memory need to be targeted in parallel."
| Contact: | Thomas Iskratsch |
| Email: | t.iskratsch@qmul.ac.uk |




