Events
Prof Zhongqiang Yang (Tsinghua University), Spontaneous formation of water droplets at oil-solid interfaces (Athena Swan Seminar Series)
Spontaneous formation of water droplets at oil-solid interfaces
| Date: | Friday 22 July 2016 12:00 - 13:00 |
| Location: | SEMS Seminar Room |
Prof Zhongqiang Yang obtained Gates Cambridge Trust and Overseas Research Studentship to do her PhD with Prof. Wilhelm T. S. Huck at the University of Cambridge, focusing on nanofined liquid crystalline materials towards nanoactuators, 2003-2007. She went to the University of Wisconsin-Madison, carrying out post-doctoral work with Prof. Nicholas L. Abbott on liquid crystal-aqueous interfaces for biological applications, 2007-2010. Since 2010, she joined the Chemistry Department at Tsinghua University as Associate Professor, working on DNA Nanotechnology. Her research interests include soft matter (such as DNA and liquid crystal) and interfaces. zyang@tsinghua.edu.cn
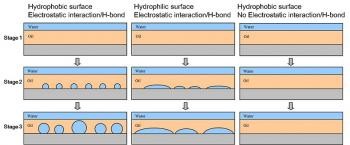
Spontaneous formation of water droplets at oil-solid interfaces
In this talk, I will share an interesting phenomenon of macroscopic water droplets spontaneously formed at oil-solid interfaces, which was accidently observed back to 2009. However, a simple thermodynamic model based on macroscopic interfacial energetic arguments fails to account for this phenomenon, which is still a mystery to scientists. In a typical experiment, a several tens micrometer thick oil layer is supported on a solid substrate and then submerged under water. The substrate can be silica, mica, quartz or glass, as long as it can form strong interaction with water via electrostatic force or hydrogen bond. Oils ranging from nematic/isotropic liquid crystals, edible oil, silicone oil, paraffin oil, to melted wax, all allow water molecules to diffuse through oil layer to the underlying solid substrates. The wettability of solid substrates determines the water spreading extent, hydrophobic surface tends to dewet water to droplets and hydrophilic surface wets water to pancake-like film. Confocal characterization verifies that water molecules nucleate at the oil-solid interface, grow and coalesce to a size up to the thickness of oil layer. The water droplets are stable for at least a couple of months. We also found that, if the bulk aqueous solution contains electrolytes (salt or sugar) above a threshold of concentration, osmotic effect sufficiently prevents water droplets formation. Interestingly, if water droplets already form, the addition of electrolytes will cause shrinkage and even vanish of water droplets. We note that tremendous studies have reported on the spontaneous formation of nanobubbles at interfaces, to a certain extent, water droplets at oil-solid interfaces are analogy to nanobubbles. However, further investigation is needed to elucidate the fundamental mechanism and directs the rational design for controlling and potentially using such interfacial phenomenon.
| Contact: | Dr Lei Su |

