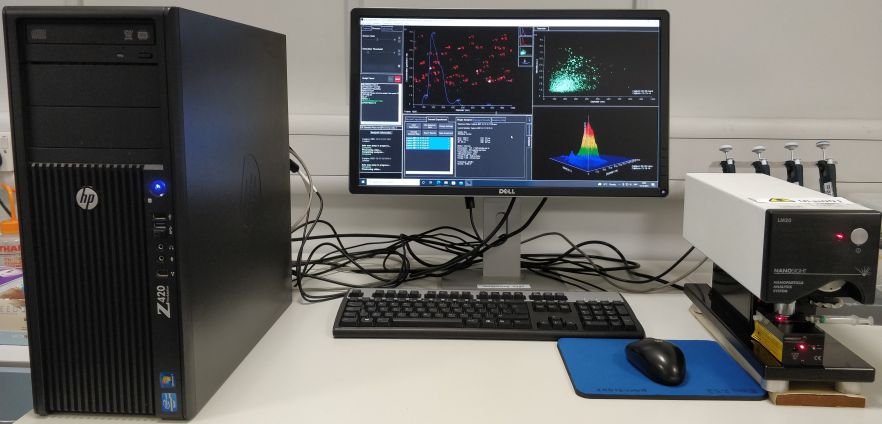
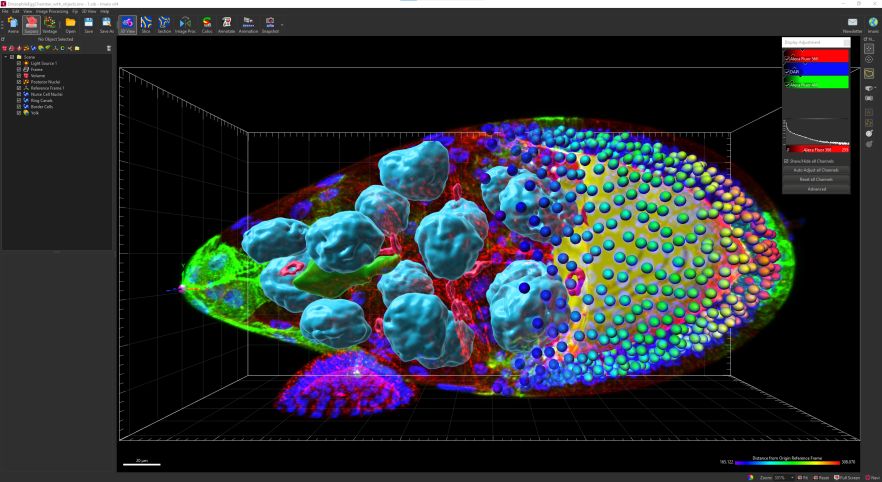
Confocal and Super-resolution Microscopy Facility
Within the Bioimaging Centre in the School of Engineering and Materials Science we have the following two confocal microscope systems: a Nikon CSU-W1 SoRa spinning disk, and a Zeiss LSM710 Elyra PS.1 super resolution system purchased through the Institute of Bioengineering, as well as two inverted epifluorescence microscopes: Leica DMi8 & Leica DMI4000B, and an incubator based Lumascope 720 for live cell imaging. We also have a Nanosight LM20 for size distribution of particles, an upright Jenapol Polarized Microscope, an iBright FL1500 for western blots and nucleic acid gels, and Imaris 9.7.2 workstation for imaging data analysis. The bioimaging facilities have contributed significantly to the publication output in SEMS and other institutes at QMUL. To apply for a training of any of our bioimaging facilities, please request through iLab.
- Zeiss LSM710 ELYRA PS.1
- Nikon CSU-W1 SoRa Spinning Disk Confocal
- Leica DMI4000B
- Leica DMi8
- Lumascope 720
- Nanosight LM20
- Jenapol Polarized Microscope
- iBright FL1500
- Imaris 9.7.2
Zeiss Super resolution LSM710 ELYRA PS.1

LSM 710 ELYRA PS.1 is a combination of an inverted laser scanning confocal microscopy 710 (LSM 710) with super resolution imaging microscopy ELYRA PS.1: photo activated localization microscopy (PALM)/Stochastic Optical Resolution Microscopy (STORM) and structured illumination microscopy (SIM). It is suitable for imaging targeting at 10-100nm resolution at single molecule level. In addition, Zen 2012 software, motorised X/Y stage, definite focus, TIRF mode make it capable of acquisition of tiling, multiple positions, time lapse, FRAP and FRET. It has a on stage environment chamber with a 35mm petri dish adaptor linked with a CO2/N2 controller for live cell imaging including hypoxic experiments. Furthermore, it comes with an offline data processing workstation.
Objectives:
- EC Plan-Neofluar10x/0.3 M27,
- EC Plan-Neofluar20x/0.5 M27,
- Plan-Apochromat 63x/1.4 Oil DIC M27,
- Plan-Apochromat 100x/1.46 Oil DIC M27
Lasers:
- LSM 710: Diode laser 405nm (30mW), Ar/ML 458/488/514nm (35mW), HeNe 543nm (1mW), HeNe 633nm (5mW)
- SIM/PALM: HR Diode 405nm (50mW), HR Diode 488nm (100mW), HR DPSS 561nm (100mW), HR Diode 642nm (150mW)
Cameras:
- pco.edge sCMOS for SIM
- Andor iXon DU 897 back-illuminated EMCCD for PALM/STORM
SOP Zeiss LSM 710 Elyra PS1 superresolution
Nikon CSU-W1 SoRa Spinning Disk Confocal

Nikon CSU-W1 SoRA Super-Resolution Spinning Disk Confocal scanning unit, couples the Nikon Ti2-E inverted microscope platform to the CSU-W1 SoRa system from Yokogawa. The system is equipped with two camera adapters, disk changer with two spinning disks (a 50 μm pinhole W1 spinning disk, and a 50 μm micro-lensed pinhole SoRa spinning disk), a full enclosure incubator with temperature, CO2 and humidity control for live imaging, FRAP and FRET. The system uses NIS-Elements software for data acquisition and image processing, and it comes with an offline data processing workstation.
Objectives:
- CFI Plan Fluor 10x/0.3/WD 16mm,
- CFI Plan Apochromat VC 20x/0.75/WD 1mm,
- CFI Super Fluor 40xC/0.9/WD 0.34-0.26mm,
- CFI Plan Apochromat Lambda D 60x/1.42/WD 0.15mm Oil
- CFI Apochromat TIRF 60xC/1.49/WD 0.12mm Oil
Lasers:
- LuxX Diode lasers: 405nm (120mW), 445nm (100mW), 488nm (200mW), 515nm (100mW), 638nm (200mW)
- Coherent OBIS LS laser 561nm (150mW)
Camera:
- Two Photometrics Prime BSI back-illuminated sCMOS cameras
SOP Nikon CSU W1 SoRa
Leica DMI4000B Epifluorescence Microscope

Leica DMI4000B is an inverted epifluorescence widefield (WF) microscope suitable for bright field (BF), phase contrast (PH) acquisition and epifluroscence imaging with DAPI, FITC, TRITC, and Texas Red filters on fixed cell and tissue samples.
Objectives:
- N PLAN 2.5x/0.07
- HCX PL FLUOTAR 10x/0.3 PH1,
- HCX PL FLUOTAR 20x/0.5 PH2,
- HCX PL APO 40x/1.25-0.754 Oil,
- HCX PL FLUOTAR 63x/1.25 Oil PH3
Camera:
- LEICA DFC9000 GT sCMOS camera for epifluorescence and BF/PH imaging
SOP Leica DMI4000B
Leica DMi8 Epifluorescence Microscope

Leica DMi8 is a semiautomatic inverted epifluorescence widefield (WF) microscope suitable for bright field (BF), phase contrast (PH) acquisition and epifluroscence 2D and 3D imaging with DAPI, GFP, Cy3, and Cy5 filters on fixed cell and tissue samples.
Objectives:
- HC PL FLUOTAR 10x/0.32 PH1,
- HC PL FL L 20x/0.40 CORR, PH1,
- HC PL FL L 40x/0.60 CORR PH2,
- HC PL FLUOTAR 63X/1.30 Oil PH3
Camera:
- LEICA DFC9000 GT sCMOS camera for epifluorescence and BF/PH imaging
SOP Leica DMi8
Lumascope 720

Lumascope 720 is an automatic incubator based, inverted 3-colour (blue, green, red) epifluorescence microscope, with motorized XY stage and auto Z-focus. It is suitable for multiple positions, time lapse live imaging with both phase contrast and fluorescence 2D and 3D mode, up to 10 fps, or 30 fps with reduced frame size.
Objectives:
- Motic EF-N Plan 4x/0.10 (not for imaging),
- Olympus CAch N 10x/0.25,
- Olympus LCAch N 20x/0.40,
- Olympus LCAch N 40x/0.55
Camera:
- CMOS camera for epifluorescence and PH imaging
SOP Lumascope 720
Nanosight LM20
Nanosight LM20 with a LM10 laser unit (Class 1 diode 635 nm) characterizes the size distribution and concentration of all types of nanoparticles from 20nm-1000nm in solution by dynamically analysing the paths the particles take under Brownian motion over a suitable period of time.
SOP Nanosight LM20
Jenapol Polarized Microscope

Jenapol is an upright polarized microscope with a MC4K 8.3MP Sony IMX334 CMOS camera, using Pixit Pro Version 3.2 software for imaging acquisition, suitable for cultured cells, tissues, mineral or other transparent material samples.
Objectives:
- Planachromat Pol 10x/0.2,
- Planachromat Pol 20x/0.4,
- Planachromat Pol 50x/0.95
Camera:
- MC4K 8.3MP CMOS camera
SOP Jenapol Polarized Microscope
iBright FL1500

iBright FL1500 Imaging System supports the main imaging applications of fluorescent, chemiluminescent, and colorimetric western blots, in addition to fluorescent stained nucleic acid gels, fluorescent stained protein gels, colorimetric stained protein gels, and colorimetric membrane stains.
Features of the iBright FL1500 Imaging System:
• 9.1 MP cooled CCD camera: high sensitivity and dynamic range to help enable the detection of subtle differences in samples
• Five fluorescence channels: multiplex and capture up to four proteins in a single blot for more meaningful and representative experiments
• Smart Exposure technology: provides rapid determination of optimal exposure time to help minimize the need to repeat exposures to acquire the desired signal
• Simple interface: clear layout of functions and features combined with a 12.1-inch capacitive touchscreen for a smooth imaging experience
• Advanced automated features: automatic sample rotation, automatic zoom, and automatic focus help streamline image capture
• 22.5 x 18.0-cm field of view: large field of view for high-throughput imaging (image up to four mini or two midi blots at a time)
• Green LED-based transilluminator: effectively excite popular DNA dyes such as ethidium bromide and Invitrogen SYBR Green dyes with an alternative to UV-based transilluminators
• Flexible connectivity: export captured images via ethernet connection, Wi-Fi (with optional accessory), USB, or directly to Connect cloud-based platform
• Multiple image analysis options: perform densitometry, quantitation, and normalization directly using the on-instrument software, or for more in-depth analysis use iBright Analysis Software (available in both desktop and cloud-based versions)
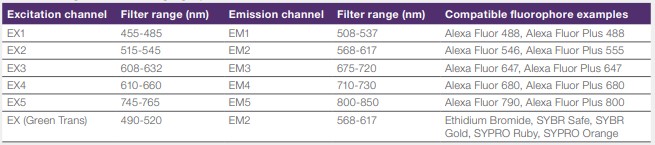
SOP iBright FL1500
Imaris 9.7.2 ----- Imaris Single Full with ClearView
Imaris Single Full gives you complete power and flexibility of all Imaris functionalities at your fingertips. Visualization of complex 3/4D microscopy datasets with automated Spots and Surfaces detection and visualisation (100s of GBs), smart detection of complex objects, tracing of neurons, blood vessels (no lumen) or other filamentous structures, tracking including cell division detection, batch analysis and a wide range of customized analysis powered by MatLab or Python. Imaris integrates ClearView™ algorithms for Spinning Disk Confocal, Widefield Fluorescence, Brightfield, Laser Scanning Confocal and TIRF, allowing GPU-accelerated Deconvolution.
SOP IMARIS
Imaris Quickstart Tutorials
Imaris Reference Manual
For more information:
https://imaris.oxinst.com/products/imaris-single-full
Online learning centre:
https://imaris.oxinst.com/learning/
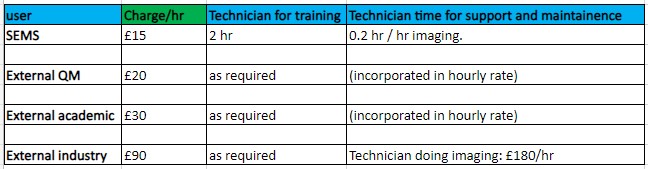
Charges
Nikon CSU-W1 SoRa, Zeiss LSM710 Elyra PS.1:
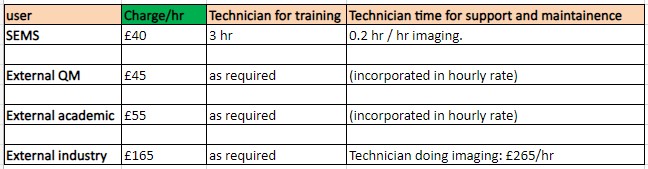
Leica DMi8, Leica DMI4000B, Lumascope 720, Nanosight LM20:
Publications
- Miao T, Symonds A, Hickman OJ, Wu D, Wang P, Lemoine N, Wang Y, Linardopoulos S, Halldén G (2024). Inhibition of Bromodomain Proteins Enhances Oncolytic HAdVC5 Replication and Efficacy in Pancreatic Ductal Adenocarcinoma (PDAC) Models. International Journal of Molecular Sciences 25(2), 1265. doi: 10.3390/ijms25021265 Impactor factor: 6.2
- Verbruggen SW, Nolan J, Duffy MP, Pearce OMT, Jacobs CR, Knight MM (2024). A Novel Primary Cilium?Mediated Mechanism Through which Osteocytes Regulate Metastatic Behavior of Both Breast and Prostate Cancer Cells. Advanced Science 11(2), 2305842. doi: 10.1002/advs.202305842 Impactor factor: 17.521
- Swiatlowska P, Tipping W, Marhuenda E, Severi P, Fomin V, Yang Z, Xiao Q, Graham D, Shanahan C, Iskratsch T (2023). Hypertensive pressure mechanosensing alone triggers lipid droplet accumulation and transdifferentiation of vascular smooth muscle cells to foam cells. Advanced Science 2308686. doi: 10.1002/advs.202308686 Impactor factor: 17.521
- Thompson CL, Hopkins T, Bevan C, Screen HRC, Wright KT, Knight MM (2023). Human vascularised synovium-on-a-chip: a mechanically stimulated, microfluidic model to investigate synovial inflammation and monocyte recruitment. Biomedical Materials 18(6), 065013. doi: 10.1088/1748-605X/acf976 Impactor factor: 4
- Nolan J, Pearce OMT, Screen HRC, Knight MM, Verbruggen SW (2023). Organ-on-a-Chip and Microfluidic Platforms for Oncology in the UK. Cancers 15(3), 635. doi: 10.3390/cancers15030635 Impactor factor: 5.2
- Meng H, Fu S, Ferreira MB, Hou Y, Pearce OM, Gavara N, Knight MM (2023). YAP activation inhibits inflammatory signalling and cartilage breakdown associated with reduced primary cilia expression. Osteoarthritis and Cartilage 31(5), 600-612. doi: 10.1016/j.joca.2022.11.001 Impactor factor: 7
- Swiatlowska P, Sit BHM, Zhen F, Marhuenda E, Xanthis I, Zingaro S, Ward M, Zhou X, Xiao Q, Shanahan C, Jones G, Yu C-H, Iskratsch T (2022). Pressure and stiffness sensing together regulate vascular smooth muscle cell phenotype switching. American Association For The Advancement of Science: Science Advances 8(15). doi: 10.1126/sciadv.abm3471 Impactor factor: 14.136
- Jones CFE, Di Cio S, Connelly JT, Gautrot JE (2022). Design of an Integrated Microvascularized Human Skin-on-a-Chip Tissue Equivalent Model. Frontiers in Bioengineering and Biotechnology, Frontiers Media 10. doi: 10.3389/fbioe.2022.915702 Impactor factor: 5.7
- Thompson C, Mcfie M, Chapple J, BEALES P and Knight M (2021). Polycystin-2 is required for chondrocyte mechanotransduction and traffics to the primary cilium in response to mechanical stimulation. International Journal of Molecular Sciences 22(9), 4313. doi: 10.3390/ijms22094313 Impactor factor: 6.2
- Raynold AAM, Li D, Chang L, Gautrot JE (2021). Competitive binding and molecular crowding regulate the cytoplasmic interactome of non-viral polymeric gene delivery vectors. Nature Communications 12 (1). doi: 10.1038/s41467-021-26695-w Impactor factor: 16.6
- Chronopoulos A, Thorpe SD, Cortes E, Lachowski D, Rice AJ, Mykuliak VV, et al. (2020). Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nature Materials 19(6), 669-678. doi: 10.1038/s41563-019-0567-1 Impactor factor: 38.663
- Torres-Pérez JV, Naeem H, Thompson CL, Knight MM, Novak P (2020). Nanoscale mapping reveals functional differences in ion channels populating the membrane of primary cilia. Cellular Physiology and Biochemistry 54(1), 15-26. doi: 10.33594/000000202 Impactor factor: 5.11
- Di Cio S, Iskratsch T, Connelly JT, Gautrot JE (2019). Contractile myosin rings and cofilin-mediated actin disassembly orchestrate ECM nanotopography sensing. Biomaterials 232. doi: 10.1016/j.biomaterials.2019.119683 Impactor factor: 12.121
- Walker RV, Keynton JL, Grimes DT, Sreekumar V, Williams DJ, Esapa C, Wu D, Knight MM, Norris DP (2019). Ciliary exclusion of Polycystin-2 promotes kidney cystogenesis in an autosomal dominant polycystic kidney disease model. Nature Communications 10(1), 4072. doi: 10.1038/s41467-019-12067-y Impactor factor: 12.121
- Okesola BO, Wu Y, Derkus B, Gani S, Wu D, Knani D, Smith DK, Adams DJ, Mata A (2019) Supramolecular self-sssembly to control structural and biological properties of multicomponent hydrogels. Chemistry of Materials 31(19):7883-7897. doi: 10.1021/acs.chemmater.9b01882 Impactor factor: 9.567
- Li W and Wang W (2019). Membrane tension regulates syndecan-1 expression through actin remodelling. Biochimica et Biophysica Acta (BBA) - General Subjects 1863(11), 129413-129413. doi: 10.1016/j.bbagen.2019.129413 Impactor factor: 4.008
- Fu S, Thompson CL, Ali A, Wang W, Chapple JP, Mitchison HM, Beales PL, Wann AKT and Knight MM (2019). Mechanical loading inhibits cartilage inflammatory signalling via an HDAC6 and IFT-dependent mechanism regulating primary cilia elongation. Osteoarthritis and Cartilage 27(7), 1064-1074. doi: 10.1016/j.joca.2019.03.003 Impactor factor: 4.68
- Rowson DT, Shelton JC, Screen HRC and Knight MM (2018). Mechanical loading induces primary cilia disassembly in tendon cells via TGFβ and HDAC6. Scientific Reports 8(1), 11107. doi: 10.1038/s41598-018-29502-7 Impactor factor: 4.576
- Wu D, Lee S, Luo J, Xia H, Gushchina S, Richardson PM, Yeh J, Krügel U, Franke H, Zhang Y, Bo X.(2018). Intraneural injection of ATP stimulates regeneration of primary sensory axons in the spinal cord. Journal of Neuroscience 38(6), 1351-1365. doi: 10.1523/JNEUROSCI.1660-17.2017 Impactor factor: 5.3
- Gushchina S, Pryce G, Yip P, Wu D, Pallier P, Giovannoni G, Baker D, Bo X (2018). Increased expression of colony-stimulating factor-1 in mouse spinal cord with experimental autoimmune encephalomyelitis correlates with microglial activation and neuronal loss. Glia 66 (10), 2108-2125. doi: 10.1002/glia.23464 Impactor factor: 5.984
- Freeley M, Attanzio A, Cecconello A, Amoroso G, Clement P, Fernandez G, Gesuele F, Palma M (2018). Tuning the Coupling in Single-Molecule Heterostructures: DNA-Programmed and Reconfigurable Carbon Nanotube-Based Nanohybrids. Advanced Science 5(10), 1800596-1800596. doi: 10.1002/advs.201800596 Impactor factor: 15.84
- Thorpe SD, Gambassi S, Thompson CL, Chandrakumar C, Santucci A, and Knight MM (2017). Reduced primary cilia length and altered Arl13b expression are associated with deregulated chondrocyte Hedgehog signaling in alkaptonuria. Journal of Cellular Physiology 232(9), 2407-2417. doi: 10.1002/jcp.25839 Impactor factor: 5.096
- Gambassi S, Geminiani M, Thorpe SD, Bernardini G, Millucci L, Braconi D, et al. (2017). Smoothened-antagonists reverse homogentisic acid-induced alterations of Hedgehog signaling and primary cilium length in alkaptonuria. Journal of Cellular Physiology 232(11), 3103-3111. doi: 10.1002/jcp.25761 Impactor factor: 5.096
- Thompson CL, Plant JC, Wann AK, Bishop CL, Novak P, Mitchison HM, Beales PL, Chapple JP and Knight MM (2017). Chondrocyte expansion is associated with loss of primary cilia and disrupted hedgehog signalling. European Cells and Materials 34, 128-141. doi: 10.22203/eCM.v034a09 Impactor factor: 3.741
- Schwarz N, Lane A, Jovanovic K, Parfitt DA, Aguila M, Thompson CL, da Cruz L, Coffey PJ, Chapple JP, Hardcastle AJ, Cheetham ME (2017). Arl3 and RP2 regulate the trafficking of ciliary tip kinesins. Human Molecular Genetics 26, 13(1), 2480–2492. doi: 10.1093/hmg/ddx143 Impactor factor: 5.1
- Wu D, Lee S, Luo J, Xia H, Gushchina S, Richardson PM, Yeh J, Krügel U, et al. (2017). Intraneural injection of ATP stimulates regeneration of primary sensory axons in the spinal cord. Journal of Neuroscience 38(6), 1351-1365. doi: 10.1523/JNEUROSCI.1660-17.2017 Impactor factor: 5.673
- Freeley M, Worthy HL, Ahmed R, Bowen B, Watkins D, Macdonald JE, Zheng M, Jones DD, et al. (2017). Site-Specific One-to-One Click Coupling of Single Proteins to Individual Carbon Nanotubes: A Single-Molecule Approach. Journal of the American Chemical Society 139(49), 17834-17840. doi. 10.1021/jacs.7b07362 Impactor factor: 14.612
- Thompson CL., Wiles, A., Poole CA. and Knight MM (2016). Lithium chloride modulates chondrocyte primary cilia and inhibits Hedgehog signaling. The FASEB Journal 30(2), 716-726. doi: 10.1096/fj.15-274944 Impactor factor: 4.966
- Sliogeryte K, Thorpe SD, Wang Z, Thompson CL, Gavara N, and Knight MM(2016). Differential effects of LifeAct-GFP and actin-GFP on cell mechanics assessed using micropipette aspiration. Journal of Biomechanics 49(2), 310-317. doi: 10.1016/j.jbiomech.2015.12.034 Impactor factor: 2.32
To request a training via iLab
Cell and TissueLab Bioimaging Training
Contact
Prof Martin Knight
email: m.m.knight@qmul.ac.uk
Tel: +44 (0)20 7882 8868
Dr Dongsheng Wu
email: dong.wu@qmul.ac.uk
Tel: +44 (0)20 7882 5051